Abstract
Background: For patients with VTE, current American Society of Hematology (ASH) guideline panel suggests using direct oral anticoagulants (DOACs) over vitamin K antagonists (VKAs) where VKAs are required to be bridged with a parenteral anticoagulant (PAC). For patients with VTE and cancer, current guidelines recommend DOACs over low molecular weight heparin (LMWH) and LMWH over unfractionated heparin (heparin) for the initial treatment of VTE. Limited evidence is available about the patterns of anticoagulant treatment for VTE in routine clinical practice of large healthcare delivery networks in the United States (US) and whether the VTE treatments are aligned with current guidelines. This study aimed to assess real-world anticoagulant treatment patterns among VTE patients using harmonized electronic health record (EHR) data from four Integrated Delivery Networks (IDNs) in the US.
Methods: This was a retrospective, longitudinal, multicenter, cohort study using harmonized EHR data from both inpatient and outpatient settings. The study population included adult patients prescribed DOACs, warfarin, and/or PAC therapy as inpatient or outpatient treatment within ≤30 days of VTE diagnosis, between June 2015 through May 2018. Data from the four IDNs was pooled to describe demographic characteristics and treatment patterns among VTE patients overall and by subgroups.
Results: A total of 10,527 patients who were treated with OACs after VTE diagnosis were included for analysis. The mean (SD) age was 61.9 (5.98) years, with 46.1% aged 65 or older. More than half (53.2%) were female, and White patients comprised the majority (74.4%), followed by African American patients (22.8%). Obese and morbidly obese patients comprised 39.1% and 16.1% of patients, respectively. Among all VTE patients, warfarin-only (n=3545; 33.7%) was the most commonly used OAC treatment, followed by warfarin + PAC (n=3128; 29.7%), rivaroxaban-only (n=1357; 12.9%), rivaroxaban + PAC (n=853; 8.1%), apixaban + PAC (n=839; 8.0%), apixaban-only (n=762; 7.2%), and Other OAC (n=357; 3.4%) (Table 1). When stratifying VTE patients by age, gender, race and BMI, some variations in OAC treatment were observed. Among both older (≥65 years) and younger (<65 years) patients, warfarin-only was most commonly used, then warfarin + PAC. Warfarin-only was more commonly used among obese (36.3%) and morbidly obese (40.4%) patients than non-obese (29.8%) patients. OAC treatment patterns were generally comparable among men and women. Among White patients, approximately equal proportions of patients received warfarin + PAC (31.9%) and warfarin-only (31.0%). However, among African-American patients, a higher proportion of patients used warfarin-only (40.9%) vs. warfarin + PAC (24.5%).
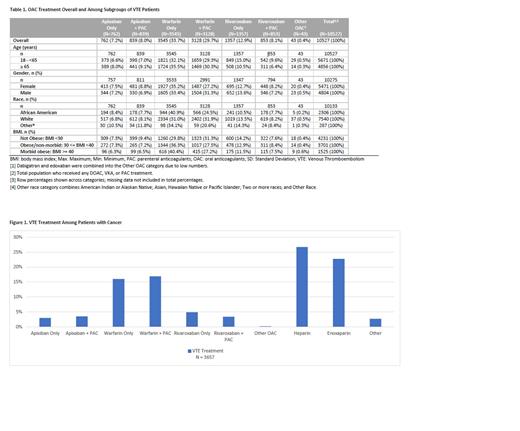
Patterns of anticoagulant treatments including OACs and/or parental anticoagulants among VTE patients with cancer were further analyzed (Figure 1). Among VTE patients with cancer (n=3657), heparin had the highest use (26.7%), then enoxaparin (22.7%); approximately the same proportion of cancer patients received warfarin-only (16.0%) and warfarin + PAC (16.9%). Of DOACs, rivaroxaban-only was the most commonly used treatment (4.9%), then apixaban + PAC (3.5%), and lastly, rivaroxaban + PAC (3.4%) among cancer patients.
Conclusion: Current VTE treatment guidelines recommend warfarin to be bridged with PAC, however, warfarin-only therapy remained the most used treatment option followed by warfarin + PAC. While rivaroxaban and apixaban are not required to be bridged with PAC, such practices were observed for a large proportion of apixaban- and rivaroxaban-treated VTE patients. VTE treatment among patients with cancer was not completely aligned with current guidelines, as heparin was more commonly used than LMWH (enoxaparin). Our findings suggest greater efforts are needed to improve anticoagulant treatment practices among VTE patients.
Dhamane: Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Rosenblatt: Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Jiang: Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Guo: Bristol Myers Squibb: Ended employment in the past 24 months. Dorsch: Agency for Health Research and Quality: Research Funding; National Institutes of Health/National Institute of Aging: Research Funding; American Health Association Health IT Research Network: Research Funding; Janssen Pharmaceuticals: Honoraria; Bristol Myers Squibb/Pfizer: Research Funding; Amgen: Research Funding. Luo: Pfizer Inc: Current Employment, Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal